Specific Heat Capacity Of Solid Nitrogen . Specific heat is closely related to the concept of heat capacity. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The phase diagram of nitrogen is shown below the table. Under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. Heat capacity is the amount of heat necessary to change the temperature of a. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and.
from www.numerade.com
Specific heat is closely related to the concept of heat capacity. Under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Heat capacity is the amount of heat necessary to change the temperature of a. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. The phase diagram of nitrogen is shown below the table.
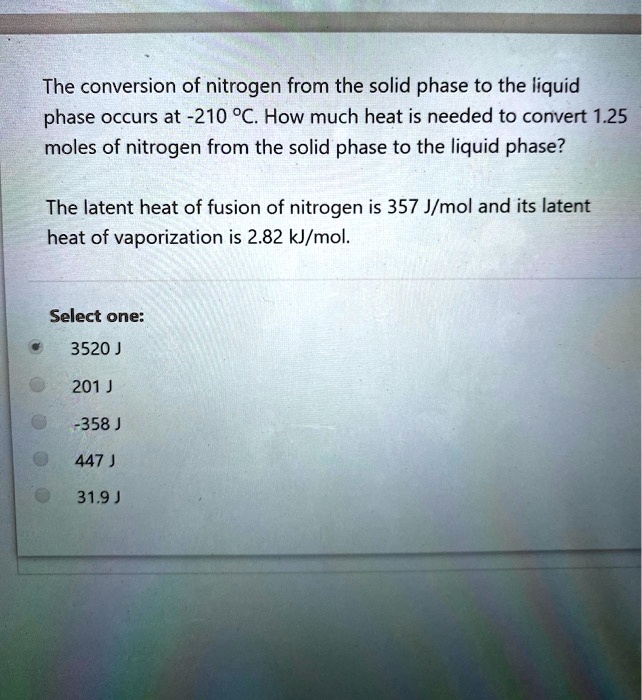
SOLVED The conversion of nitrogen from the solid phase to the liquid
Specific Heat Capacity Of Solid Nitrogen 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Heat capacity is the amount of heat necessary to change the temperature of a. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the. The phase diagram of nitrogen is shown below the table. Specific heat is closely related to the concept of heat capacity. Under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket.
From physicsomets.netlify.app
Physics Formula For Specific Heat Capacity Specific Heat Capacity Of Solid Nitrogen Heat capacity is the amount of heat necessary to change the temperature of a. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. Under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. 55 rows the table of specific heat capacities gives the volumetric. Specific Heat Capacity Of Solid Nitrogen.
From studylib.net
Heat Capacity of Metals PreLab Specific Heat Capacity Of Solid Nitrogen Specific heat is closely related to the concept of heat capacity. Heat capacity is the amount of heat necessary to change the temperature of a. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount. Specific Heat Capacity Of Solid Nitrogen.
From www.learnpick.in
Specific Heats Of Solids Physics PowerPoint Slides LearnPick India Specific Heat Capacity Of Solid Nitrogen 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of. Specific Heat Capacity Of Solid Nitrogen.
From www.slideserve.com
PPT Unit 09 PowerPoint Presentation, free download ID5621270 Specific Heat Capacity Of Solid Nitrogen The phase diagram of nitrogen is shown below the table. Specific heat is closely related to the concept of heat capacity. Under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. 55 rows the table. Specific Heat Capacity Of Solid Nitrogen.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas Heat Of Formation Specific Heat Capacity Of Solid Nitrogen 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Specific heat is closely related to the concept of heat capacity. The phase diagram of nitrogen is shown below the table. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of. Specific Heat Capacity Of Solid Nitrogen.
From www.tutorix.com
To determine specific heat capacity of given solid by method of mixtures Specific Heat Capacity Of Solid Nitrogen In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the. Specific heat is closely related to the concept of heat capacity. The phase diagram of nitrogen is shown below the table. Heat capacity is the amount of heat necessary to change the temperature. Specific Heat Capacity Of Solid Nitrogen.
From www.sliderbase.com
Specific Heat Specific Heat Capacity Of Solid Nitrogen In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The phase diagram of nitrogen is shown below the. Specific Heat Capacity Of Solid Nitrogen.
From www.slideserve.com
PPT Heat capacity and Specific Heat PowerPoint Presentation, free Specific Heat Capacity Of Solid Nitrogen Under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. Specific heat is closely related to the concept of heat capacity. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the. The specific heat (= specific heat capacity) at. Specific Heat Capacity Of Solid Nitrogen.
From www.researchgate.net
Specific heat in constant volume of the nitrogen versus temperature in Specific Heat Capacity Of Solid Nitrogen Heat capacity is the amount of heat necessary to change the temperature of a. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the. Specific heat is closely related to the concept of heat capacity. Under prolonged exposure to fire or heat, nitrogen. Specific Heat Capacity Of Solid Nitrogen.
From universe-review.ca
Atoms Specific Heat Capacity Of Solid Nitrogen Heat capacity is the amount of heat necessary to change the temperature of a. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the. The phase diagram of nitrogen is shown below the table. Under prolonged exposure to fire or heat, nitrogen containers. Specific Heat Capacity Of Solid Nitrogen.
From galvinconanstuart.blogspot.com
Nitrogen Phase Diagram Pressure Temperature General Wiring Diagram Specific Heat Capacity Of Solid Nitrogen Specific heat is closely related to the concept of heat capacity. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. In thermodynamics, the specific heat capacity (symbol c) of a substance. Specific Heat Capacity Of Solid Nitrogen.
From nanohub.org
Courses Introduction to Materials Science & Engineering Specific Heat Capacity Of Solid Nitrogen The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. Under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass. Specific Heat Capacity Of Solid Nitrogen.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Heat Capacity Of Nitrogen Gas Specific Heat Capacity Of Solid Nitrogen In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the. Heat capacity is the amount of heat necessary to change the temperature of a. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat. Specific Heat Capacity Of Solid Nitrogen.
From www.shutterstock.com
Find Specific Heat Given Solid By Stock Vector (Royalty Free Specific Heat Capacity Of Solid Nitrogen 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Heat capacity is the amount of heat necessary to change the temperature of a. The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. In. Specific Heat Capacity Of Solid Nitrogen.
From brainly.in
Specific heat capacity of the substance is decreasing with increase in Specific Heat Capacity Of Solid Nitrogen 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. Heat capacity is the amount of heat necessary to change the temperature of a. Specific heat is closely related to the concept of heat capacity. The phase diagram of nitrogen is shown below the table. In. Specific Heat Capacity Of Solid Nitrogen.
From byjus.com
The SI unit of specific heat capacity of a substance is Specific Heat Capacity Of Solid Nitrogen Heat capacity is the amount of heat necessary to change the temperature of a. 55 rows the table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and. The phase diagram of nitrogen is shown below the table. Under prolonged exposure to fire or heat, nitrogen containers may rupture violently. Specific Heat Capacity Of Solid Nitrogen.
From www.tec-science.com
Specific heat capacity of selected substances tecscience Specific Heat Capacity Of Solid Nitrogen Heat capacity is the amount of heat necessary to change the temperature of a. Under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the. Specific heat is closely related. Specific Heat Capacity Of Solid Nitrogen.
From www.youtube.com
To Find the Specific Heat Capacity of Solid by Using Method of Mixtures Specific Heat Capacity Of Solid Nitrogen The specific heat (= specific heat capacity) at constant pressure and constant volume processes, and the ratio of specific heats and. Heat capacity is the amount of heat necessary to change the temperature of a. Under prolonged exposure to fire or heat, nitrogen containers may rupture violently and rocket. 55 rows the table of specific heat capacities gives the volumetric. Specific Heat Capacity Of Solid Nitrogen.